Latest Version
Version
2.0.1
2.0.1
Update
May 17, 2025
May 17, 2025
Developer
Food and Drug Administration
Food and Drug Administration
Categories
Education
Education
Platforms
Android
Android
Downloads
1
1
License
Free
Free
Package Name
gov.fda.obook
gov.fda.obook
Report
Report a Problem
Report a Problem
More About OB Express 2.0
*** Important note: We regret to inform you our mobile app will no longer be available as of 10/03/2024. In its place, we are excited to offer you a responsive web design option via the Orange Book.
The Orange Book website provides a seamless experience, including access to all our features and services, online and on your mobile devices. You may access the site in its entirety at the following location: https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm. ***
We appreciate your understanding and support during this transition.
============================================
New in this version:
• Search results and drug listings now clarify which listed drugs are reference listed drugs (RLDs) and which are reference standards.
• The application now uses HTTPS encryption to provide a secure connection.
With FDA’s Orange Book Express app, it’s now faster and easier to find information about:
• Drug products approved on the basis of safety and effectiveness by the Food and Drug Administration (FDA)
• Information about patents and exclusivity
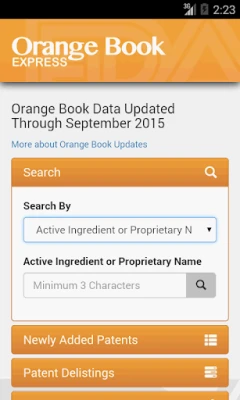
Orange Book Express allows you to:
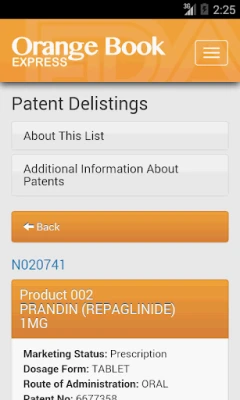
• Search by Active Ingredient or Proprietary Name; Applicant Holder (Firm); Application Number; or Patent Number.
• Search all marketing statuses (Rx, OTC, Discontinued) with one search.
• Look up patent and exclusivity information.
• Identify Reference Listed Drugs (RLDs) and determine if a drug product has a therapeutic equivalent.
• Browse Patent Delistings and Newly Added Patents.
We appreciate your understanding and support during this transition.
============================================
New in this version:
• Search results and drug listings now clarify which listed drugs are reference listed drugs (RLDs) and which are reference standards.
• The application now uses HTTPS encryption to provide a secure connection.
With FDA’s Orange Book Express app, it’s now faster and easier to find information about:
• Drug products approved on the basis of safety and effectiveness by the Food and Drug Administration (FDA)
• Information about patents and exclusivity
Orange Book Express allows you to:
• Search by Active Ingredient or Proprietary Name; Applicant Holder (Firm); Application Number; or Patent Number.
• Search all marketing statuses (Rx, OTC, Discontinued) with one search.
• Look up patent and exclusivity information.
• Identify Reference Listed Drugs (RLDs) and determine if a drug product has a therapeutic equivalent.
• Browse Patent Delistings and Newly Added Patents.
Rate the App
Add Comment & Review
User Reviews
Based on 0 reviews
No reviews added yet.
Comments will not be approved to be posted if they are SPAM, abusive, off-topic, use profanity, contain a personal attack, or promote hate of any kind.
More »










Popular Apps

Decor Matters: Home Design AppDecorMatters, Inc.

K-LOVE On DemandEducational Media Foundation

Bus Simulator BangladeshGhost Interactive

Tripadvisor: Plan & Book TripsTripadvisor

History HitHit Networks Ltd

SecondScreenBraden Farmer
RYZE BangladeshBanglalink

myNoise | Focus. Relax. Sleep.myNoise BV

Planet Matters: Clean Up, EarnPlanet Matters

Kasa SmartTP-LINK SYSTEMS INC.
More »










Editor's Choice

ZEPETO: Avatar, Connect & LiveNaver Z Corporation

ArtStationArtStation
Smartify: Arts and CultureSmartify CiC

Ritual Urban RetreatRitual Urban Retreat Inc.

Suite MobileTurbo - Groupe BPCE

Truth or Dare Game - Party AppChouic

AI Voice: Text to Speech TTSVoiser Teknoloji Limited Sirketi

Automotive DictionaryPocket Dictionary

ArcSiteArcSite

Ingress PrimeNiantic Spatial Inc